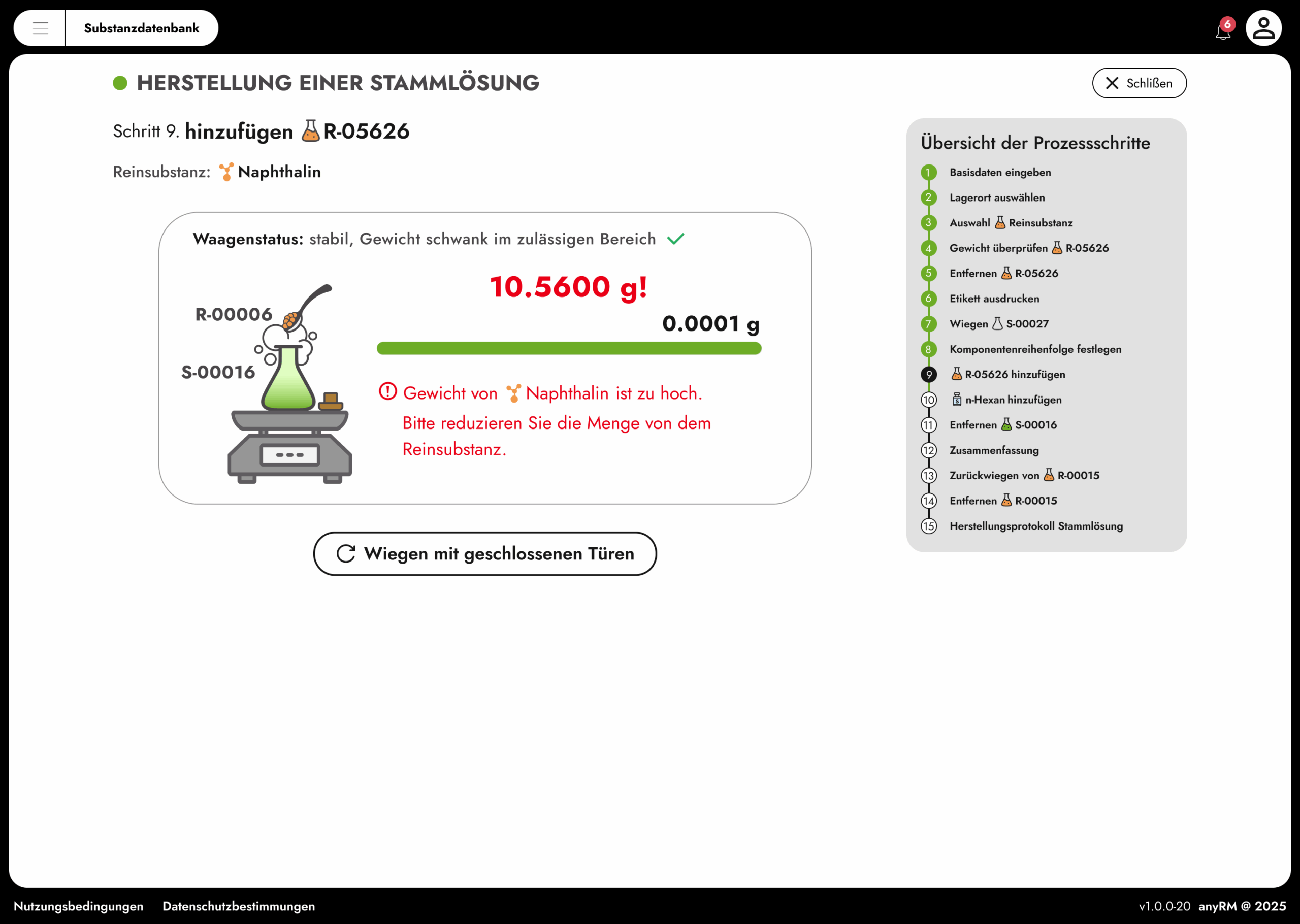
Thanks to our integrated audit trail function, you maintain full control over your system in accordance with your requirements or the 17025 standards. This ensures complete traceability, clear responsibilities, and regulatory compliance. In this way, you can consistently maintain the quality and consistency of your laboratory processes at all times.
+49 (0)30 120 8 32 021
Panoramastr. 1, 10178 Berlin